Abstract
Background:
There is compelling rationale to use bridging radiotherapy (BRT) between leukapheresis and chimeric antigen receptor T-cell infusion for cytoreduction and palliation of relapsed/refractory B cell malignancies. There is also preclinical evidence suggesting possible immune synergy with the combination of BRT and CAR T. Existing BRT series are small and generally focused on diffuse large B-cell lymphoma (DLBCL). We report a large cohort of RT-bridged patients including early experience with BRT for non-DLBCL histologies.
Methods:
We analyzed 43 patients with DLBCL (n=35), mantle cell lymphoma (MCL; n=6), and Burkitt's lymphoma (BL; n=2). All received RT for any intent within 1 month prior to leukapheresis up until CAR T infusion. PET response was evaluated by Lugano criteria within the BRT treatment field ("in field"), outside of the BRT field ("out of field") and overall at 5 time points: pre-BRT, pre-CAR T infusion, and day +30 (n=41), +90 (n=30), and +180 (n=19). Survival analysis was per Kaplan-Meier and clincodemographic associations with survival were assessed by Cox univariate proportional hazards.
Results:
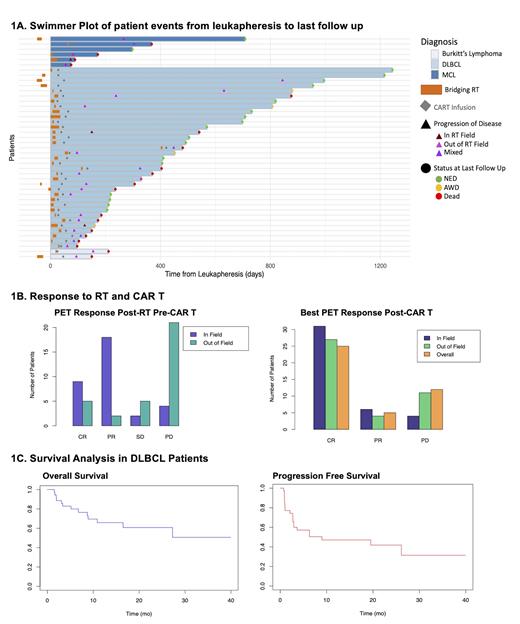
Patients received axicabtagene (n=20, 48%), tisagenlecleucel (n=11, 26%), lisocabtagene (n=8, 19%), brexucabtagene (n=2, 5%), and an experimental CAR T product (n=1, 2%). Overall, patients had a median of 3 prior therapies (range 1-9). Prior to BRT, most (74%) had advanced stage III/IV relapsed/refractory disease and 17 (40%) had bulky (>7.5 cm) lesions. There was heterogeneity in BRT-treatment parameters; most commonly treated sites were head/neck (n=10), pelvis/groin (n=8), and abdomen (n=7). Median BRT dose was 30 Gy (range 4-54), with 20 Gy in 5 fractions as the most common regimen (n=12). 18 (42%) received BRT to all sites of PET avid disease ("comprehensive BRT"). 16 (37%) patients also received systemic bridging therapy, including concurrently (n=10) with BRT.
With a short 14d median time to restaging PET, in field overall response rate (ORR) post BRT was 82% (complete response, CR; n=9, 27% or partial response, PR; n=18, 55%) [Figure 1]. Reflective of the population referred for bridging, 21 (64%) had out of field progression post BRT and pre-CAR T. Post-CAR T, 2 patients (5%) had grade 3 cytokine release syndrome, 4 (9%) had grade 3 neurotoxicity, and no severe toxicity within the BRT field was noted.
Following CAR T, ORR was 72% (CR: n=25, 60%; PR: n=5, 12%). Median post CAR T follow-up was 9 mos (range 1-40), with 44% (n=19) of patients having no evidence of disease, 44% (n=19) deceased, and 12% (n=5) alive with disease at last contact. 33 (77%) patients never progressed within the BRT field. Of the 27 (63%) patients who relapsed, 10 were CD19- and 9 (33%) had disease in the BRT field at first progression.
In DLBCL patients, progression free survival (PFS) was 83%, 60%, and 51% and overall survival (OS) was 100%, 89% and 69%, respectively at Day +30, +90, and +180. Univariate analysis showed that pre-BRT ECOG performance status ≥2 (HR: 12.0, p<0.005), CNS disease (HR: 7.6, p=0.006), 3 prior systemic therapies (HR: 8.1, p=0.05), and BRT dose 30 Gy (HR: 0.21, p=0.01) were significantly associated with OS. Timing of BRT relative to leukapheresis was not a significant predictor of PFS or OS.
Conclusion:
BRT has broad utility for aggressive B-cell malignancies and is associated with excellent pre-CAR T local control and no serious toxicity within the irradiated sites. Most patients had durable post-CAR T local control. Prospective studies are planned to clarify outcomes and evaluate mechanistic synergies. Future studies are needed to define optimal patient characteristics for systemic vs. radiotherapy bridging and elucidate the best BRT strategy for maximizing local control and potential immune augmentation.
Palomba: Kite: Consultancy; Novartis: Consultancy; Notch: Honoraria, Other: Stock; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; Lygenesis: Honoraria; PCYC: Consultancy; BeiGene: Consultancy; Pluto: Honoraria; Wolters Kluwer: Patents & Royalties; Juno: Patents & Royalties; Ceramedix: Honoraria; Magenta: Honoraria; Priothera: Honoraria; Nektar: Honoraria; WindMIL: Honoraria; Rheos: Honoraria. Shouval: Medexus: Consultancy. Batlevi: Regeneron: Current holder of individual stocks in a privately-held company; Viatris: Current holder of individual stocks in a privately-held company; TouchIME: Honoraria; BMS: Current holder of individual stocks in a privately-held company; Moderna: Current holder of individual stocks in a privately-held company; Pfizer: Current holder of individual stocks in a privately-held company; Medscape: Honoraria; TG Therapeutics: Consultancy; Seattle Genetics: Consultancy; Life Sciences: Consultancy; Kite Pharma: Consultancy; Dava Oncology: Honoraria; Karyopharm: Consultancy; Juno/Celgene: Consultancy; Bayer: Research Funding; Memorial Sloan Kettering Cancer Center: Current Employment; ADC Therapeutics: Consultancy; GLG Pharma: Consultancy; Xynomic: Research Funding; Roche/Genentech: Research Funding; Novartis: Research Funding; Epizyme: Research Funding; Janssen: Research Funding; Autolus: Research Funding. Brentjens: BMS: Consultancy, Patents & Royalties, Research Funding; Gracell Biotechnologies, Inc: Consultancy, Ended employment in the past 24 months; sanofi: Patents & Royalties; Caribou: Patents & Royalties. Dahi: Kite / Gilead: Membership on an entity's Board of Directors or advisory committees. Giralt: JENSENN: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees; Actinnum: Membership on an entity's Board of Directors or advisory committees. Park: Kura Oncology: Consultancy; Intellia: Consultancy; Autolus: Consultancy; Artiva: Consultancy; Novartis: Consultancy; Amgen: Consultancy; PrecisionBio: Consultancy; Curocel: Consultancy; Affyimmune: Consultancy; BMS: Consultancy; Minerva: Consultancy; Servier: Consultancy; Kite Pharma: Consultancy; Innate Pharma: Consultancy. Scordo: i3 Health: Other: Speaker; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; Omeros Corporation: Consultancy; Angiocrine Bioscience: Consultancy, Research Funding; McKinsey & Company: Consultancy. Sauter: Gamida Cell: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Genmab: Consultancy; GSK: Consultancy; Bristol-Myers Squibb: Research Funding; Spectrum Pharmaceuticals: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding. Shah: Janssen: Research Funding; Amgen: Research Funding. Perales: NexImmune: Honoraria; Kite/Gilead: Honoraria, Other; Karyopharm: Honoraria; Bristol-Myers Squibb: Honoraria; Cidara: Honoraria; Incyte: Honoraria, Other; Equilium: Honoraria; Celgene: Honoraria; MorphoSys: Honoraria; Medigene: Honoraria; Omeros: Honoraria; Novartis: Honoraria, Other; Nektar Therapeutics: Honoraria, Other; Miltenyi Biotec: Honoraria, Other; Merck: Honoraria; Sellas Life Sciences: Honoraria; Servier: Honoraria; Takeda: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal